I am sorry that this must be my last SRP blog post.
My final presentation will take place May 10 at 7:00 p.m.
My MIC and Biofilm tests have generally shown that dilute concentrations of dishwashing detergents do not greatly affect bacteria of the human gut. Although the MIC tests showed significant reductions in Lactobacillus, 10mg/mL seems to be a rather large concentration that free-floating bacteria would come into contact with in the body. While there were still reductions in bacterial counts for the biofilm tests for both 0.1 and 1 mg/mL detergent, they were not so large as to be particularly concerning.
Additionally, the detergent tests showed that certain biofilms were washed away rather than killed and that these detergents could just move flora from one part of the gut to another. Therefore, based on my data and what we already know about human gut flora, consuming small quantities of dishwashing detergent shouldn't be too bad for you after all. However, until we find out more about the role of gut flora, it will be hard to determine whether the "swept away" biofilms would have any effect on our health.
In the 1980s, this scientist by the name of Stratchan hypothesized that the cleaning products we use were essentially making us sick by killing bacteria that would give us immunity to certain diseases. While it may be true upon further investigation using more toxic detergents and cleaning products (such as those containing the chemical triclosan), it does not appear to correlate with the data I have collected throughout this project.
However, to add to this experiment, it would be ideal first to complete my data set on 1 mg/mL detergent against Lactobacillus delbrueckii( using a live culture of course) and further investigate the topic as a whole. What would happen to the biofilms if you used toothpaste? We swallow toothpaste all the time, after all. One could investigate the effects of other products, such as window cleaners, or test the biofilms when grown on different plates. This kind of investigation could ultimately go in may directions, from just investigating gut flora to looking to remove biofilms from medical equipment ( a lot of research and effort has already been directed toward preventing biofilm growth on surgical implants and equipment). All in all, there are many new possibilities and continuations this investigation to follow, and science always leaves us with more questions.
But to close, I wanted to point out what a valuable experience this was for me. When I first was offered the opportunity to investigate this aspect of Microbiology and work in Dr.Koppisch's lab, I was interested, but still wondered how any of this bacterial stuff could affect me. I initially wanted to go down to Phoenix and shadow a doctor or witness a surgery, and I wasn't sure if this project would be fulfilling. But it truly, truly was.
Despite the challenges of learning to work in a new kind of lab, with new techniques, procedures and precautions, I learned not only that microbiology was fascinating, but also the value of the "little things" in life (no pun intended). Sometimes you just have to stop riding your ego and see that even the things that many people would consider "boring' or "juvenile" may actually be some of the things that are the most important. Microbes may be small, but they are essential to our survival. I may have not been working with any super corrosive acids or toxic chemicals, but those dish soaps give us an insight into how the products we use in our daily lives affect our health.
I am honored to have worked in the Koppisch lab for the last couple months, and I have enjoyed the majority of my time in the lab, working with what I see as a little world at my fingertips, the little creatures that seem so small and insignificant, but also can have such a big impact on everything we do. From weighing down ships to contaminating surgical tubing, these biofilms truly do affect our lives.
While I still want to eventually be a physician, working in microbiology has definitely sparked my interest, and I look forward to taking some classes in college. And this experience, I believe, has truly helped prepare me for what lies ahead. Once again, I would like to thank Dr.Koppisch and his students for guiding me along the way and for not also being my colleagues, but also my friends.
Спасибо за
прочитать. (Thank you for reading)
Спасибо , преподаватель Koppisch и ваших студентов. (Thank you, Dr.Koppisch and your students.)
я буду скучать по вам! (I will miss you guys!)
Mackenzie
(P.S. If you are interested in
learning how to pronounce any of the Russian gibberish I've been throwing at
you, I will be launching a website this summer called pronouncerus.weebly.com
that, despite my nascent knowledge of the language, should help some of you
curious folks learn some words.)






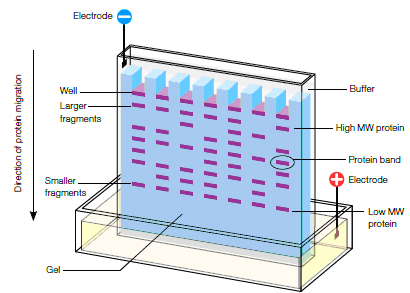 (Image credit: http://www.bio-rad.com/webroot/web/images/lsr/solutions//technologies/protein_electrophoresis_blotting_and_imaging/protein_electrophoresis/technology_detail/pet11_img1.jpg)
(Image credit: http://www.bio-rad.com/webroot/web/images/lsr/solutions//technologies/protein_electrophoresis_blotting_and_imaging/protein_electrophoresis/technology_detail/pet11_img1.jpg)







